List Of Mineral Inorganic Acid
All mineral acids form hydrogen ions and the conjugate base when dissolved in water. A list of the mineral acids includes.
Aluminium oxide Al 2 O 3.

List of mineral inorganic acid. Commonly used mineral acids are sulfuric acid hydrochloric acid and nitric acid they are also known as bench acids citation needed. The formula for each hydrohalic acid you are likely to encounter is given. The Contact Process for the manufacture of sulphuric acid.
An inorganic compound is a substance that does not contain both carbon and hydrogenA great many inorganic compounds do contain hydrogen atoms such as water H 2 O and the hydrochloric acid HCl produced by your stomach. There are strong inorganic acids like HCl HNO 3 H 2 SO 4 and weak inorganic acids like HCN or H 2 S. Aluminium carbide Al 4 C 3.
Inorganic acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals both organic and inorganic. Oxygen acids with oxygen. Hydroiodic acid also known as hydriodic acid HClO 4.
Aluminium arsenate AlAsO 48H 2 O. Inorganic acids range from superacids such as perchloric acid HClO 4 to very weak acids such as boric acid H 3 BO 3. Carboxylic acid is a function and.
ActiniumIII oxide - Ac 2 O 3. Loba chemie offers wide range of Inorganic acids such as acids of great strength like sulfuric acid to very weak - boric acid. The mineral acids include the bench acidshydrochloric acid sulfuric acid and nitric acidso-called because they are the acids most commonly used in a laboratory setting.
Mineral acids range from superacids perchloric acid to very weak. Commonly used inorganic acids are sulfuric acid H 2 SO 4 hydrochloric acid HCl and nitric acid HNO 3. These materials are generally amino acid complexes proteinates chelates polysaccharide complexes and propionates.
Aluminium arsenide AlAs. Aluminium iodide AlI 3. Inorganic acids are also called mineral acids.
They are free of carbon. See also Acid-base properties of aqueous solutions of salts with ions from both acids and bases Buffer solutions pKa of amines diamines and cyclic organic nitrogen compounds. In contrast only a handful of inorganic compounds contain carbon atoms.
Mineral Acid Definition and List. This means inorganic acid compounds can donate H ions to the aqueous solution or can accept electron pairs from electron rich compounds. Minerals are naturally found in an inorganic state ie.
Hydrogen Halides pKa 317 -80 -90 for HF HCl HBr Boric Acid pKa 923 Nitric Acid pKa -13. Note that the chemical symbol for hydrogen H is written before the chemical symbol of the Group 17 element. Inorganic acid or mineral acid is an acid derived from one or more inorganic compounds All mineral acids form hydrogen ions and the conjugate base ions when dissolved in water.
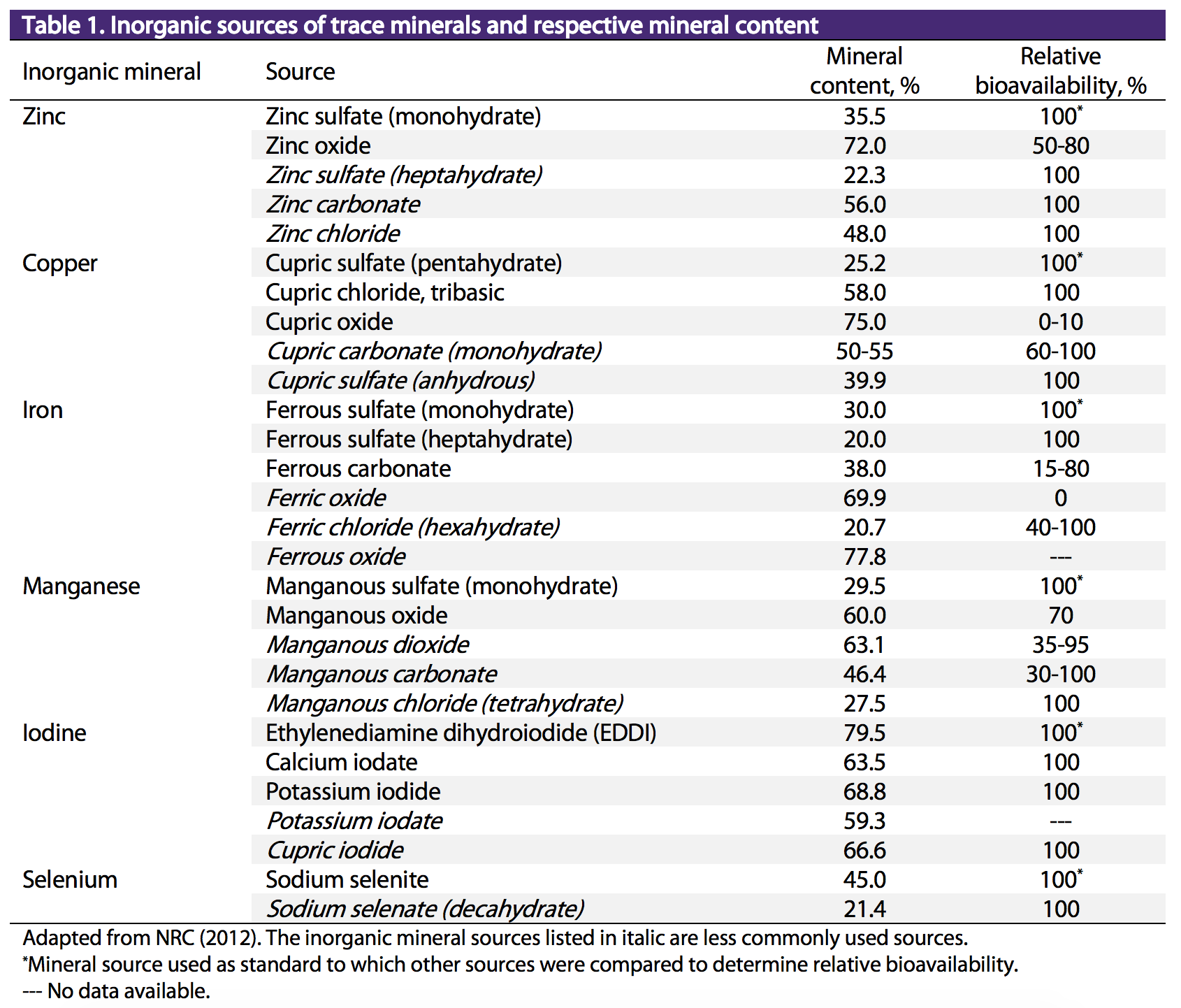
Conversely the trace mineral in an inorganic supplement has been combined with an inorganic salt such as zinc carbonate zinc sulfate and zinc chloride. Helmenstine Anne Marie PhD. Sulfuric Acid is an Inorganic Acid Inorganic acids release protons when dissolved in water.
At the heart of the Contact Process is a reaction which converts sulphur dioxide into sulphur trioxide. ActiniumII fluoride - AcF 3. Aluminium nitride AlN.
Some inorganic acids have oxygen atoms in their structure whereas some inorganic acids do not. ActiniumIII chloride - AcCl 3. Sulphur dioxide gas is passed together with air as a source of oxygen over a solid vanadiumV oxide catalyst.
Based on the above information some of the key differences between organic acid and inorganic acids are as follows. This process of binding a mineral with an organic molecule is called chelation pronounced ke-lay-shun. PK a values given in the table are measuered at 25C unless other temperatureC is indicated with superscript at the pKa value.
A mineral acid or inorganic acid is an acid derived from one or more inorganic compounds. A synonym for inorganic acids is mineral acids and they originate from mineral sources. The general molecular formula for all hydrohalic acids is HX aq where H is the symbol for hydrogen X represents the symbol for one of the halogen elements and aq indicates that this is an aqueous solution.
Inorganic acids are the most commonly used acids in the laboratories some of which are as follows. Hydrogen acids without oxygen formed by hydrogen and another compound or a polyatomic anion. Inorganic acids tend to be very soluble in water and insoluble in organic solvents.
The Sigma-Aldrich portfolio of inorganic acids spans products with various acidity pKa values measured in water. Definitions of the acid dissociation constant and pK a are given below the table. When an inorganic mineral is combined with an amino acid molecule think protein it creates an amino acid chelate.
Organic simply means that the mineral is bound to an organic material. Inorganic acids fall into two broad categories. The carbonic acid is a mineral acid but has a coal the difference is not carbon itself but carbon bonds see.
H 2 SO 4. Aluminium boride AlB 2. Aluminium bromide AlBr 3.
This is therefore an example of heterogeneous catalysis. For example H2SO4 is an inorganic acid having oxygen atoms. Hydrochloric acid HCL Nitric acid HNO3 Phosphoric acid H3PO4 Sulphuric acid H2SO4 Boric acid H3BO3 etc.
Aluminium antimonide AlSb.
 Guide To Vitamins And Minerals How To Get Your Mineral Nutrition Micronutrients Mineral Chart
Guide To Vitamins And Minerals How To Get Your Mineral Nutrition Micronutrients Mineral Chart
 Difference Between Mineral Acids And Organic Acids Compare The Difference Between Similar Terms
Difference Between Mineral Acids And Organic Acids Compare The Difference Between Similar Terms
 Pin On Vitamins And Minerals Chart
Pin On Vitamins And Minerals Chart
 Difference Between Organic Acid And Inorganic Acid Compare The Difference Between Similar Terms
Difference Between Organic Acid And Inorganic Acid Compare The Difference Between Similar Terms
 Pin On Vitamins And Minerals Chart
Pin On Vitamins And Minerals Chart
 Nomenclature Naming Polyatomic Ions Study Chemistry Inorganic Compound Nomenclature Chemistry
Nomenclature Naming Polyatomic Ions Study Chemistry Inorganic Compound Nomenclature Chemistry
 Organic Chemistry Quotes Biotechnology Organic Chemistry Jokes Organic Chemistry Chemistry Jokes
Organic Chemistry Quotes Biotechnology Organic Chemistry Jokes Organic Chemistry Chemistry Jokes

Post a Comment for "List Of Mineral Inorganic Acid"